Noncoding RNAs and Energy Homeostasis
"Put simply, the conundrum is this: Only 1.2% of the genome encodes proteins, but 80% is transcribed into RNA. So either the genome is replete with useless transcription or these noncoding RNAs fulfill some unexpected function! What was dismissed as junk because it was not understood may well turn out to hold the secrets to human complexity and a guide to the programming of complex systems in general!" (J. Mattick)
The impact of noncoding RNAs (ncRNAs) like microRNAs and long, noncoding RNAs (lncRNAs) on eukaryotic gene regulation has just started to be understood. The Kornfeld lab takes part in this 'Noncoding Revolution' by identifying novel ncRNAs and elucidating their role in homeostatic circuits regulating energy homeostasis in health and disease-associated processes.
We apply state-of-the-art techniques and technologies, such as next-generation sequencing, the generation of transgenic mouse models, and in vivo anti-ncRNA loss-of-function, to better understand the intricate biology of disease-associated ncRNAs.
Our ultimate goal is to identify sequence-specific ncRNAs depletion approaches for anti-obesity intervention.
Research interests
(1) MicroRNAs and Glucose Metabolism
MicroRNAs are small, 21-22nt-long nCRNAs that modulate stability and translational capability of cognate mRNA target(s). According to recent prediction algorithms, mammalian genomes encode for >1000 microRNAs, with each microRNA targeting up to 100s of protein-coding transcript. More than 60% of all mammalian mRNAs are predicted to be regulated by microRNA-evoked post-transcriptional inhibition. Thus, the regulatory spectrum of microRNA-based gene regulation is enormous.
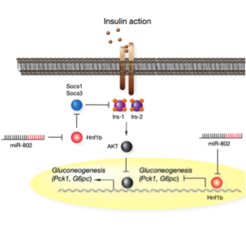
Whereas most studies have concentrated on transcriptional regulation of glucose metabolism, a large conceptual void still remains regarding the role of post-transcriptional gene silencing by microRNAs in control mechanisms of energy metabolism in healthy as well as obesity-associated diseases like type 2 diabetes mellitus (T2D). We have recently identified and characterized a glucoregulatory microRNA, namely miR-802, and demonstrated that obesity-associated rises in miR-802 deteriorate hepatic glucose metabolism via silencing of the diabetes risk gene hepatocyte nuclear factor 1 beta (Hnf1b). High levels of miR-802-Hnf1b lead to uncurbed glucose production, hyperglycemia, and impairment of glucose homeostasis in mice and, presumably, human T2DM patients. The identification of tissue-specific and disease-associated microRNAs and efficacy of recently introduced microRNA inhibitors offers the unique prospect of designing microRNA loss-of-function regimens for better treatment of obesity and T2D.
(2) LncRNAs and Glucose Homeostasis
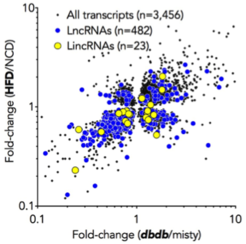
LncRNAs are a diverse class of ncRNAs with a broad spectrum of gene regulatory functions. Due to their tissue-specific and dynamic expression, lncRNAs were recently paraphrased as 'dark matter of the genome'. In this project, we ask whether lncRNAs control glucose metabolism and are involved in obesity-associated loss of insulin sensitivity in liver – the major glucoregulatory organ in mammals. Using RNA-Seq and novel lncRNA prediction algorithms, we observed that obesity alters expression of 28 annotated and 15 hitherto unknown lncRNAs in two mouse models of obesity and insulin resistance. To identify lncRNAs causally involved in the control of glucose metabolism, we established a siRNA screening system that allows functional interrogation of >600 lncRNAs. These in vitro findings will serve as an entry point for the generation of lncRNA knockout mice.
(3) Noncoding RNAs and Adipose Tissue Plasticity
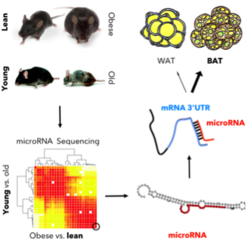
Throughout the industrialized world, the obesity pandemic poses a significant health burden with more than 300 million adults diagnosed as clinically obese (WHO). Weight intervention approaches often include reductions in caloric intake and surgical interventions. Such approaches often fail to significantly lower body weight due to metabolic adaptations such as reductions in energy expenditure and food intake. However, opposing approaches aiming at increasing basal metabolic rates, and thus, the turnover of ingested calories, are less stringently accompanied by metabolic adaptations. Hence, they harbor a high potential as a future anti-obesity treatment. In this context, activation of brown adipose tissue (BAT) has recently moved into focus as another potential anti-obesity treatment. This potential is exemplified by the intriguing fact that seemingly negligible amounts of brown fat significantly increase energy expenditure and basal metabolic rates. Moreover, it has been estimated that as a little as 50 g of additional BAT would combust 20% more of calories ingested per day in humans. Thus, identifying novel regulatory ncRNAs that control BAT differentiation, activation, and function is of critical importance. Our ultimate goal is to define novel sequence-specific anti-ncRNAs therapeutics for anti-obesity intervention.
To this end, we have recently identified a shortlist of microRNAs and lncRNAs correlating with BAT differentiation and activation, and are currently generating tailored transgenic mouse models to better understand their in vivo function in controlling glucose metabolism.


