Research
Research Group Fenselau
An organism’s ability to tightly coordinate energy balance and glucose metabolism is critical for metabolic health and survival. Such coordination is achieved by multiple distinct neuronal cell populations that sense energy-related signals and relay this information to downstream brain sites to orchestrate diverse processes such as feeding behaviour, energy expenditure and glucose homeostasis. To achieve this, it is essential that communication within these circuits is efficient and highly precise as well as appropriately adapted to energy and nutrient availability.
Our research focuses on advancing our current understanding of synapse physiology and plasticity – such as synaptic long-term potentiation – in circuits that regulate systemic metabolism. The overarching goal of our research is to establish a causal relationship between synaptic transmission in defined circuits and biological processes at the cellular, tissue and organismal levles. Specifically, we are addressing the following three research areas:
Deciphering the synaptic organization of neural gut-to-brain communication
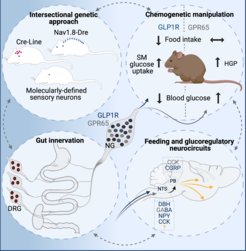
The gastrointestinal tract constitutes a major information source regarding available nutrients. Given its ability to rapidly digest and absorb food, information about the nutritive content must be promptly relayed to the brain in order to limit excessive food intake and to prime the body for changes in nutrient availability. Peripheral sensory neurons that innervate the organs of the gastrointestinal tract represent an important element of this gut-to-brain communication. These afferent neurons synaptically transmit nutrient-related signals from the gastrointestinal tract organs to downstream neurons in the central nervous system. This gut-to-brain communication contributes significantly to the regulation of feeding behaviour and glucose metabolism.
Recent studies have shown that molecularly distinct sensory neurons innervate different tissues of the gastrointestinal tract and respond to different gut-derived signals. However, the identity of the sensory neuron populations that participate in the regulation of food intake and glucose metabolism regulation along with the pertaining downstream circuits in the brain remain poorly understood. Therefore, the overarching aims of our studies in this research area is are to determine the functional role of gut-innervating sensory neuron subtypes and to elucidate the properties of their communication to downstream neurons. A major obstacle in deciphering the functional circuits has been the technical difficulties associated with cell-type-specific targeting of sensory neurons. To overcome these issues, we have recently designed a dual-recombinase genetic approach that allows mapping and manipulating molecularly defined sensory neurons (Borgmann et al., Cell Metab., 2021). Subsequent studies have revealed that glucagon-like peptide-1 receptor (Glp1r)-expressing vagal afferents relay anorexigenic signals to control meal termination and blood glucose levels. Gut-innervating, Gpr65-expressing vagal afferents, on the other hand regulate hepatic glucose production, but they are dispensable for feeding regulation. Thus, distinct gut-innervating sensory neurons differentially control feeding and glucoregulatory neurocircuits, which may provide new ideas for the development of specific targets for metabolic control.
Defining the functional significance of synaptic plasticity in feeding circuits
Adjust responsiveness of neurocircuits to environmental changes is critical for appropriate adaptations of an organism. Synaptic plasticity is a key feature of neurocircuits to adapt in an experience-dependent manner. In our studies, we employ modern neuroscience approaches, such as optogenetics, combined with brain slice electrophysiology, to investigate aspects of synaptic plasticity in defined circuits that play a key role in regulating hunger, satiety and food preferences.
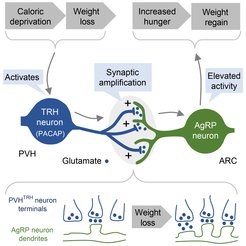
We recently revealed a crucial role for activity-dependent, remarkably long-lasting, synaptic potentiation in a defined hypothalamic circuit in the long-term control of hunger drive (Grzelka et al., Cell Metab, 2023). Specifically, we found that amplification of excitatory synaptic activity between paraventricular hypothalamus thyrotropin-releasing (PVH-TRH) neurons and hunger-promoting AgRP neurons of the arcuate nucleus causally relates to the regain of lost body weight. Silencing PVH-TRH neurons inhibited the potentiation of excitatory input to AgRP neurons and diminished concomitant regain of lost body weight upon fasting. Conversely, stimulation of this hunger circuit triggered an NMDAR-dependent gaining of body weight that persisted. Together, our findings uncovered an activity-dependent plasticity mechanism in the hypothalamus in sustaining hunger drive following weight loss, which is a major issue in obesity treatment.
Unravelling the regulatory role of neuropeptides in metabolic pathways
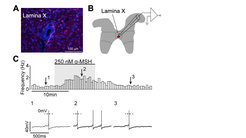
A plethora of neurotransmitters and neuropeptides have been identified in key hypothalamic and brainstem neurons that control metabolism. However, how these neuroactive chemicals operate in defined circuits remains largely enigmatic. Using optogenetic and imaging approaches combined with electrophysiology, we probe the circuit-specific action of neurotransmitters and neuropeptides. In the context of our studies, we recently demonstrated that satiety-promoting POMC neurons have long-range projections into spinal cord regions harbouring V2a interneurons, which are crucial components of premotor networks (Reinoß et al., Curr. Biol., 2020). Further, we showed that the POMC neuron-derived neuropeptide α-MSH excites these downstream interneurons and that acute inhibition of POMC neurons reduces locomotor activity. Together, our findings provide detailed insights into the synaptic communication between hypothalamic melanocortin neurons and spinal neurons, thereby providing a direct anatomical and functional link between metabolic homeostasis and locomotor control systems.


